NASBA (Nucleic Acid Sequence-Based Amplification): Principle, Innovations, and Applications
Introduction
Molecular biology has revolutionized how we detect and study RNA viruses, pathogens, and gene expression. While PCR and RT-PCR dominate the nucleic acid amplification landscape, Nucleic Acid Sequence-Based Amplification (NASBA) stands out as a powerful, isothermal amplification technique tailored specifically for RNA targets.
NASBA operates at a constant temperature of around 41 °C, making it ideal for rapid, sensitive, and equipment-light detection particularly in point-of-care diagnostics and field applications.
1. Principle of NASBA
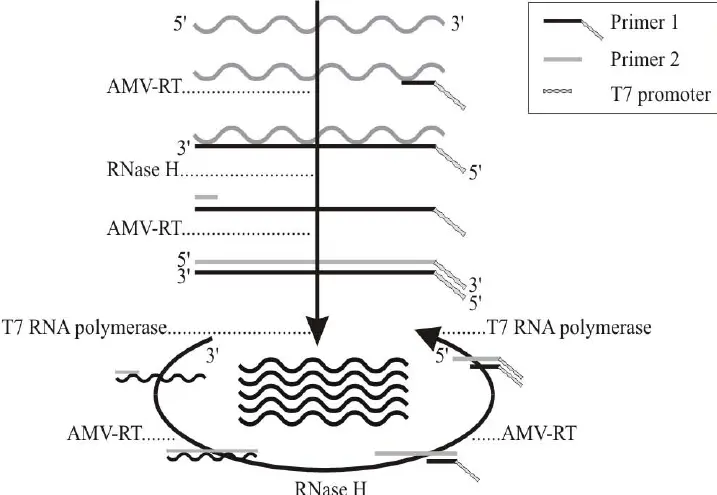
Unlike PCR, which relies on high-temperature denaturation, NASBA uses three enzymes to selectively amplify RNA without thermal cycling:
Key Enzymes in NASBA
-
Reverse Transcriptase (RT)
Converts RNA into complementary DNA (cDNA). -
RNase H
Degrades the RNA strand in RNA-DNA hybrids, leaving single-stranded DNA. -
T7 RNA Polymerase
Transcribes large amounts of RNA from the cDNA template, creating new RNA copies.
Step-by-Step Workflow
-
Primer Binding – Two primers are designed:
- Primer 1 contains a T7 promoter sequence at its 5′ end.
- Primer 2 binds downstream on the target RNA.
- Reverse Transcription – RT uses Primer 1 to synthesize cDNA from the RNA template.
- RNA Degradation – RNase H removes the original RNA strand.
- Second-Strand Synthesis – Primer 2 binds to the cDNA, and RT synthesizes double-stranded DNA.
- RNA Amplification – T7 RNA polymerase binds to the promoter sequence and generates many RNA copies.
- Cycle Continuation – Each new RNA can serve as a template, producing exponential amplification.
2. Advantages of NASBA
- Isothermal Process – No thermal cycler needed; reactions run at ~41 °C.
- RNA-Specific – Directly amplifies RNA without requiring DNA contamination removal.
- High Sensitivity – Detects down to a few copies of target RNA.
- Rapid Turnaround – Results in under two hours.
- Field-Friendly – Works with portable, battery-powered devices.
3. Innovations in NASBA Technology
In recent years, researchers have enhanced NASBA to improve performance and expand its applications:
A. Real-Time NASBA (RT-NASBA)
- Uses fluorescent molecular beacons or SYBR Green to monitor amplification in real time.
- Allows quantitative detection similar to qPCR.
B. Multiplex NASBA
- Multiple primer sets in a single reaction for simultaneous detection of different RNA targets.
- Useful in syndromic panels for respiratory viruses or sexually transmitted infections.
C. Digital NASBA
- Partitioning NASBA reactions into droplets or nano-wells for absolute quantification.
- Reduces false positives and improves precision.
D. NASBA-CRISPR Hybrid Platforms
- Combining NASBA with CRISPR-Cas13 detection for ultra-sensitive pathogen assays.
- Enables single-molecule sensitivity and rapid visual readouts.
E. Portable NASBA Devices
- Lab-on-a-chip platforms integrating sample preparation, NASBA amplification, and detection.
- Deployed for Ebola, Zika, and COVID-19 surveillance in remote areas.
4. Applications of NASBA
A. Infectious Disease Diagnostics
-
HIV-1 RNA load monitoring Early adoption in clinical virology.
- Tuberculosis diagnostics Detecting Mycobacterium tuberculosis RNA transcripts.
- Tropical disease surveillance NASBA kits for malaria, dengue, and Zika.
B. Food Safety Testing
- Detection of foodborne pathogens like Listeria monocytogenes, Salmonella, and Campylobacter from contaminated products.
C. Environmental Monitoring
- Detection of viral RNA in wastewater for epidemiological tracking.
- Monitoring harmful algal bloom toxins through RNA marker detection.
D. Veterinary Diagnostics
- Detection of Foot-and-Mouth Disease Virus (FMDV) and avian influenza in livestock and poultry.
7. Future Perspectives
The future of NASBA is moving toward:
- Fully automated, sample-to-result devices for clinics and field stations.
- NASBA coupled with CRISPR for rapid multiplex pathogen panels.
- Integration with smartphones for readout and data sharing in low-resource settings.
- Synthetic biology optimization to reduce enzyme costs and reaction times.
Conclusion
NASBA remains a cornerstone in RNA-specific amplification technologies, offering simplicity, portability, and high sensitivity.
From early HIV monitoring to real-time pandemic surveillance, NASBA has proven its value in clinical diagnostics, environmental testing, and field-based pathogen detection. As innovations continueespecially with CRISPR and lab-on-chip integration—NASBA is poised to remain a key player in the future of molecular diagnostics.