In the world of molecular biology, understanding gene expression is key to unlocking biological functions, disease mechanisms, and cellular behaviors. But what happens when the available RNA is limited, degraded, or comes from rare or difficult-to-collect samples?
This is where RNA amplification and quantitative PCR (qPCR) work hand in hand. These two technologies form a powerful combination that enables researchers to analyze even the most challenging RNA samples turning small or damaged inputs into meaningful biological insights.
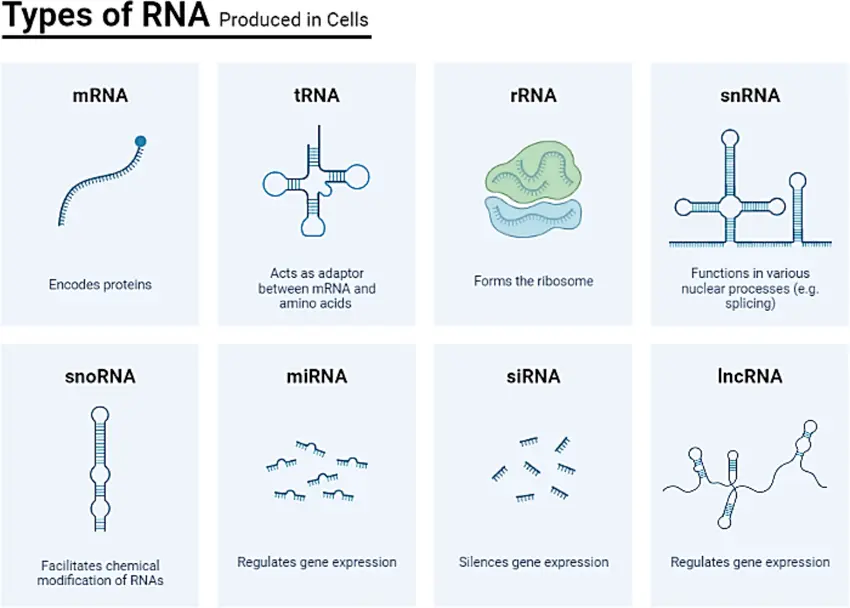
What is RNA Amplification?
RNA amplification is a molecular technique used to increase the amount of RNA in a sample before downstream analysis. This process is especially critical when working with small quantities of RNA, such as those obtained from single cells, needle biopsies, formalin-fixed tissues, or environmental samples.
RNA naturally degrades quickly and is often available only in limited quantities. Amplifying it allows scientists to recover and study gene expression patterns that would otherwise be impossible to detect. The main goal is to increase the RNA signal without introducing bias or altering the original transcript ratios.
How RNA Amplification Works
The amplification process usually starts with total RNA or mRNA extracted from a sample. Specialized enzymes are used to synthesize complementary DNA (cDNA), which is then amplified through either linear or exponential methods. Some techniques focus on enriching messenger RNA (mRNA) while minimizing the presence of ribosomal RNA (rRNA), which can otherwise dilute the meaningful data.
Linear amplification, in particular, is preferred for its ability to preserve the relative abundance of transcripts making it ideal for gene expression studies. It ensures that the data reflect real biological differences, not artifacts introduced by the amplification process.
RNA amplification is also designed to handle a wide range of input types, including degraded RNA from FFPE tissue, microbial RNA from environmental samples, or ultra-low input from single cells. It makes research possible in both human and animal biology, clinical diagnostics, and biodiversity studies.
What is Quantitative PCR (qPCR)?
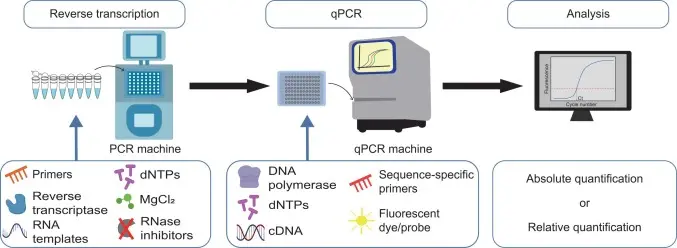
Quantitative PCR, also known as real-time PCR or RT-qPCR when working with RNA, is one of the most widely used methods for measuring gene expression. It allows scientists to quantify specific RNA sequences in real time as the amplification reaction progresses.
Before performing qPCR, RNA is typically reverse transcribed into cDNA. The PCR reaction then amplifies this cDNA using specific primers and fluorescent markers. As the amplification proceeds, the fluorescence increases proportionally to the amount of target cDNA giving precise, real-time measurements of gene expression levels.
qPCR is valued for its sensitivity, accuracy, and speed. It can detect even small changes in gene expression and is commonly used in medical research, diagnostics, environmental biology, agriculture, and veterinary science.
This technique is highly specific, meaning that researchers can focus on individual genes or panels of interest. Whether studying gene regulation, cellular responses, or biomarkers, qPCR delivers fast and reliable data often in under an hour.
Why Combine RNA Amplification with qPCR?
While qPCR is incredibly sensitive, it still requires a minimum threshold of RNA input to deliver reliable results. In many cases, especially when working with limited or compromised samples, this minimum is not met. That’s where RNA amplification becomes essential.
By amplifying RNA before performing qPCR, researchers can extend the power of gene expression analysis to virtually any sample, no matter how small or degraded.
For example, scientists working with:
- Single cells from brain tissue
- Small biopsies from animals or humans
- Field-collected samples from remote locations
- Archival clinical specimens preserved in paraffin
- Bacterial RNA without poly-A tails
The Latest Advances in qPCR Technology
As the demand for fast, precise, and scalable gene expression tools grows, qPCR technologies continue to evolve. Over the past two years, several innovations have made this technique more accessible and powerful than ever.
1. Faster Run Times
New thermocyclers can complete qPCR reactions in less than 20 minutes without sacrificing accuracy. This is particularly useful in clinical settings or when processing many samples in parallel.
2. Improved Multiplexing
Modern qPCR kits now support multiplex reactions, where multiple genes are analyzed in a single run. This saves time and material while providing a broader view of biological activity.
3. Digital PCR Convergence
qPCR platforms increasingly integrate with digital PCR capabilities, offering even higher precision and sensitivity. This is ideal for detecting rare transcripts or subtle expression changes.
4. Enhanced Primer Design Tools
Artificial intelligence and machine learning are being used to optimize primer and probe design, improving specificity and reducing the risk of false results. These tools adapt to new genome annotations and emerging variants.
5. Better Compatibility with Amplified RNA
New enzyme systems and buffer formulations are optimized to work with amplified RNA-derived cDNA, ensuring consistent performance even with low-input or degraded starting material.
Conclusion: Expanding the Frontiers of Gene Expression Analysis
The ability to analyze gene expression from small, complex, or degraded RNA samples has transformed modern biology. By combining RNA amplification with quantitative PCR, scientists can now extract rich, accurate data from material that would have previously been unusable.
This powerful combination continues to support discoveries in medicine, agriculture, neuroscience, microbiology, and beyond for both human and animal research.